
More than 30% of patients with epilepsy exhibit drug resistance and are classified as having refractory epilepsy. Surgical resection or ablation of epileptogenic network (EN) remains one of the most effective curative strategies for these patients, with precise delineation of EN being the critical determinant of surgical success. However, the intrinsic heterogeneity of epileptic boundaries poses a major technical bottleneck for accurate localization in neurosurgery. Current localization approaches suffer from substantial limitations: intraoperative electrocorticography (ECoG) is susceptible to anesthetic interference and offers limited spatial resolution; MRI and PET primarily provide preoperative structural or functional imaging and are ill-suited for real-time intraoperative navigation; meanwhile, existing molecular probes generally exhibit insufficient sensitivity, suboptimal specificity, and limited brain penetration.
To address these challenges, the research team led by Cong Wang from School of Pharmaceutical Sciences developed a cascade-amplified ratiometric fluorescent imaging strategy that enables long-term, high–signal-to-noise visualization of EN. By synergistically monitoring two core pathological hallmarks associated with abnormal epileptic discharges—acidosis fluctuations and neuroinflammation—the probe integrates a time-accumulative signal amplification mechanism with EEG-“decoupled” precision recognition, achieving stable and high-contrast imaging of EN (Figure 1). This work was published as a Research Article entitled “Cascade-Amplified Ratiometric Fluorescent Probe for Time-Locked Imaging Epilepsy” in Advanced Materials. Across four distinct epilepsy models in mice and rats, the probe consistently demonstrated robust lesion delineation. Compared with the clinical “gold standard” electrode-based EEG techniques, the probe achieved an average 25% increase in lesion-to-background contrast, over tenfold improvement in spatial resolution, and a 40.9% reduction in residual positive surgical margins, performing effectively in both cortical and deep brain lesions. Probe-guided surgical intervention resulted in an overall > 67% reduction in seizure burden.
In summary, this study introduces a logic-driven ratiometric fluorescence amplification mechanism and reports a biodegradable, BBB-penetrant polymeric fluorescent probe termed CAR-FM. The probe exhibits several key advantages: (1) High sensitivity, CAR-FM responds to subtle pH fluctuations in a “transistor-like” manner, with a response threshold below 0.2 pH units. The ratiometric fluorescence signal undergoes multiplicative cascade amplification, exceeding 150-fold. (2) Long-term imaging capability: By overcoming reliance on transient intraoperative electrical signals, CAR-FM enables high–signal-to-noise imaging (SNR > 3) for up to 100 hours, providing stable and sustained real-time guidance for surgical resection. (3) Convenient quantitative acquisition: A single-excitation, dual-emission ratiometric imaging mode allows accurate quantitative analysis from a single excitation event, minimizing environmental interference and simplifying intraoperative implementation. This work establishes a fundamentally new strategy for epilepsy surgery, with potential to improve surgical outcomes for patients with drug-resistant epilepsy. Beyond enhancing the precision of EN localization, the integrated chemical design presented here offers a conceptual framework for developing next-generation precision neurodiagnostic and neurotherapeutic systems based on molecular synergism.
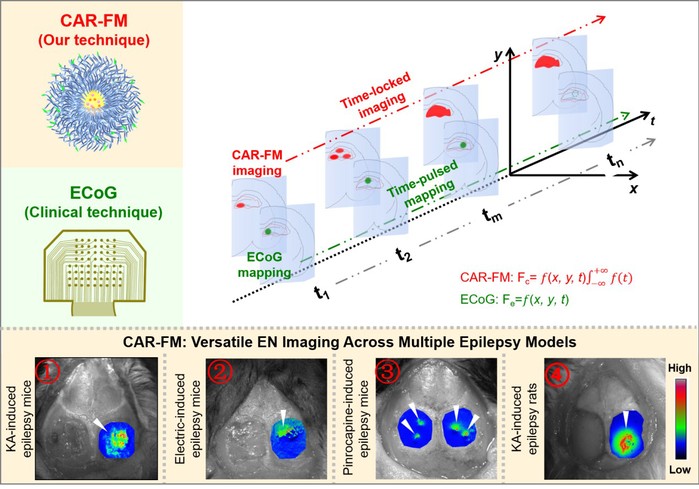
Figure 1. Cascade-amplified ratiometric fluorescent probe for epilepsy imaging.
PhD candidates Xin Wang, Bohan Li, Min Li, Yuncan Chen (School of Pharmaceutical Sciences, Fudan University), and Yurui Tang (Institute of Brain-Inspired Intelligence, Fudan University) are co–first authors of this work. Young Investigator Cong Wang and Xiao Zhu, Profeossor Cong Li from School of Pharmaceutical Sciences, Fudan University; Professor Zhen Cheng from Shanghai Institute of Materia Medica, Chinese Academy of Sciences; and Professor Xiaoyu Li from Zhongshan Hospital, Fudan University, are corresponding authors. This research was supported by National Key R&D Program of China, National Natural Science Foundation of China, Shanghai Rising-Star Program, Fudan University Medical–Engineering Integration Program, and the Open Project of the National Key Laboratory of High-End Drug Formulations for Overcoming Delivery Barriers.
Original article:
//advanced.onlinelibrary.wiley.com/doi/10.1002/adma.202514336